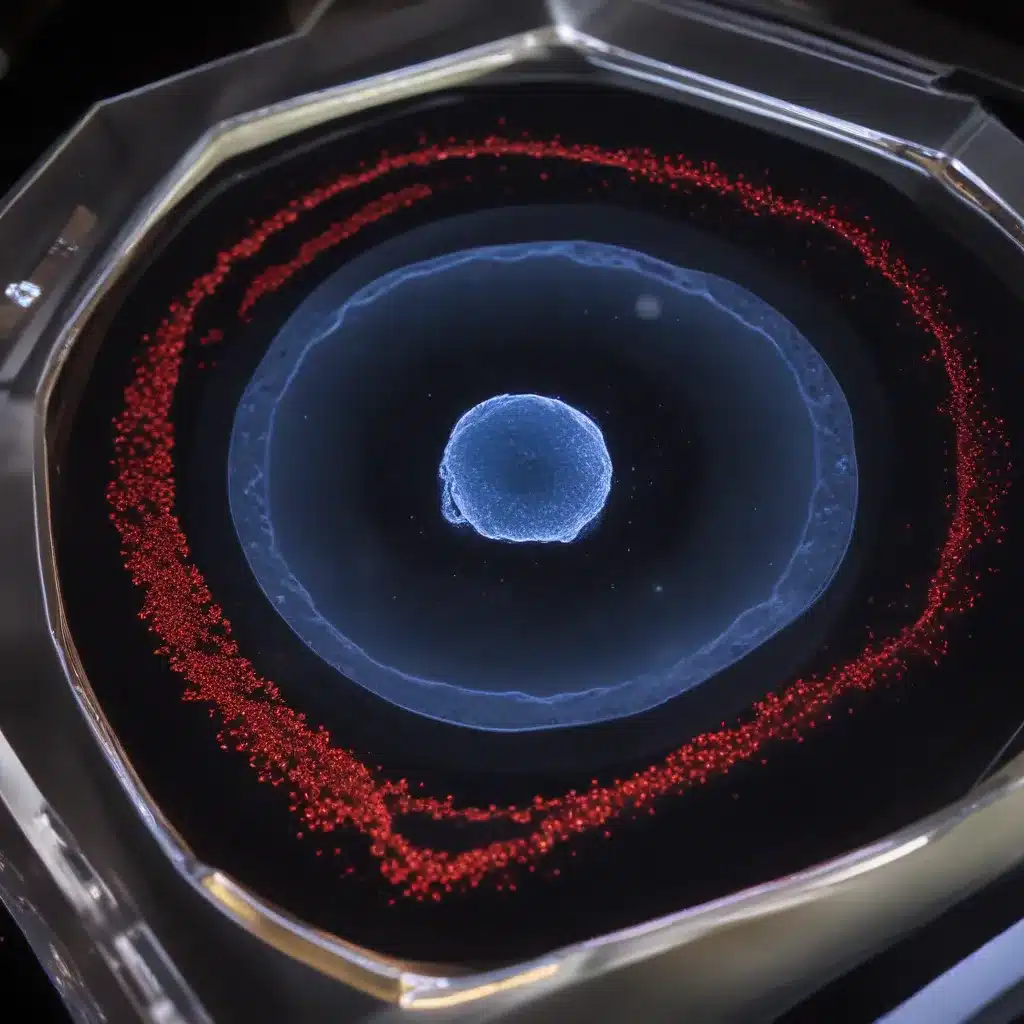
Capturing the dynamic processes of living tissues and organisms is a fundamental pursuit in modern biology and biomedical research. However, the inherent opacity and structural complexity of many biological samples poses significant challenges for high-resolution fluorescence microscopy. Optical clearing techniques have emerged as a powerful solution to enhance image quality and tissue penetration in fixed specimens, but their application to live imaging has remained limited due to concerns over sample toxicity.
In this article, we explore the revolutionary potential of a new generation of optical clearing media that are designed to be minimally invasive for live cell and tissue imaging. We will examine the principles behind these technologies, discuss their practical advantages, and consider the limitations and future directions of this exciting field.
Fundamentals of Live Cell Imaging
Before delving into the specifics of optical clearing, let’s first establish a basic understanding of the core concepts in live cell microscopy.
Principles of Live Cell Microscopy
The primary goal of live cell imaging is to visualize the dynamic cellular and subcellular processes that govern the function of living organisms, from individual cells to entire tissues and organs. This is typically achieved through the use of fluorescence microscopy, where fluorescent labels are introduced to tag specific structures or molecules of interest.
Live cell imaging presents several unique challenges compared to fixed sample imaging. Samples must be maintained in a physiologically relevant environment, with appropriate temperature, pH, and nutrient conditions. Phototoxicity and photobleaching effects from repeated illumination must also be carefully managed to ensure the viability and integrity of the living specimen.
Fluorescence Labeling Techniques
A wide range of fluorescent probes and genetically encoded tags have been developed to visualize diverse cellular structures and signaling events in live cells. Common fluorescent labels include small molecule dyes, fluorescent proteins, and quantum dots. The choice of labeling strategy depends on factors such as the target of interest, the required spatiotemporal resolution, and the potential for intervention or perturbation of the live sample.
Sample Preparation Considerations
Proper sample preparation is crucial for successful live cell imaging. Cells or tissues must be mounted in a physiologically compatible medium that maintains their structural and functional integrity. This often involves the use of agarose gels, hydrogels, or specialized cell culture media to immobilize and support the living specimen.
Optical Clearing Strategies
One of the key challenges in live cell imaging is overcoming the inherent opacity of biological samples, which can significantly degrade image quality and limit the depth of imaging. This is where optical clearing techniques come into play.
Optical Clearing Mechanisms
Optical clearing is based on the principle of refractive index matching, where the difference in refractive indices between the sample and the surrounding medium is minimized. This helps to reduce light scattering and aberrations, allowing for deeper tissue penetration and improved image resolution.
Optical clearing agents typically work by modifying the chemical composition and physical properties of the extracellular matrix and intracellular structures, effectively “clearing” the tissue and rendering it more transparent.
Optical Clearing Agents and Properties
A variety of optical clearing agents have been developed, each with their own unique properties and applications. These include polyethylene glycol (PEG), glycerol, urea, and sucrose, among others. The choice of clearing agent depends on factors such as the sample type, the desired degree of clearing, and the compatibility with downstream imaging techniques.
Tissue Preparation for Optical Clearing
Preparing samples for optical clearing often involves a series of processing steps, such as fixation, dehydration, and incubation in the clearing agent. The specific protocols can vary depending on the sample type and the clearing agent used. It is crucial to ensure that the sample preparation does not compromise the viability and functionality of the living specimen.
Advantages of Optical Clearing in Live Cell Imaging
The application of optical clearing strategies to live cell imaging has the potential to unlock a wealth of new possibilities and insights.
Enhanced Tissue Penetration
One of the primary benefits of optical clearing is the ability to achieve deeper tissue penetration and improve the visualization of structures and processes in the interior of complex, multilayered samples. This can greatly expand the scope and depth of live cell imaging, enabling the exploration of phenomena that were previously inaccessible.
Improved Image Quality
By minimizing refractive index mismatches, optical clearing can significantly enhance the quality of fluorescence images, reducing spherical aberrations and improving the signal-to-noise ratio. This translates to higher spatial resolution, better contrast, and enhanced detail in the captured images.
Applications in 3D Cell Culture
The advent of 3D cell culture models, such as organoids and spheroids, has revolutionized the study of tissue development, disease modeling, and drug screening. Optical clearing can be particularly valuable in these applications, allowing for the non-invasive, high-resolution imaging of the complex 3D architectures and dynamic processes within these systems.
Considerations and Limitations
While optical clearing holds great promise for live cell imaging, it is essential to consider the potential drawbacks and limitations of these technologies.
Biocompatibility and Cytotoxicity
One of the primary concerns with the use of optical clearing agents in live cell imaging is their potential for cytotoxicity and impact on cell viability. The chemical composition and physicochemical properties of the clearing agents must be carefully evaluated to ensure they are minimally disruptive to the living specimen.
Reversibility and Temporal Effects
The reversibility of optical clearing is another important consideration, as the clearing process should not permanently alter the structure or function of the living cells and tissues. It is crucial to understand the temporal dynamics of the clearing process and its potential impact on the sample over extended imaging sessions.
Imaging Modality Compatibility
Optical clearing techniques must be compatible with the specific imaging modalities and equipment used for live cell imaging. This may involve considerations such as refractive index matching, objective lens compatibility, and fluorescence emission spectra.
Emerging Trends and Future Directions
The field of live cell imaging with optical clearing is a rapidly evolving landscape, with exciting new developments on the horizon.
Novel Optical Clearing Techniques
Researchers are continuously exploring innovative clearing agents and methods to push the boundaries of live cell imaging. This includes the development of minimally invasive, reversible, and multi-modal clearing strategies that can be seamlessly integrated into diverse live imaging applications.
Multimodal Imaging Approaches
The integration of optical clearing with complementary imaging techniques, such as electron microscopy, x-ray imaging, and magnetic resonance imaging, can provide a comprehensive, multiscale understanding of cellular and tissue architecture and function.
Computational Image Analysis Advancements
Advances in image processing and computational analysis algorithms are crucial for extracting meaningful insights from the vast amounts of data generated by live cell imaging with optical clearing. Emerging techniques, such as deep learning and high-throughput image analysis, hold the potential to revolutionize the way we interpret and understand live biological systems.
By addressing the challenges and limitations of live cell imaging, optical clearing technologies are poised to become an indispensable tool in the arsenal of modern biological and biomedical research. As these techniques continue to evolve and be adopted more widely, we can expect to see a wealth of new discoveries and advancements in our understanding of living organisms, from the cellular to the organismal scale.



